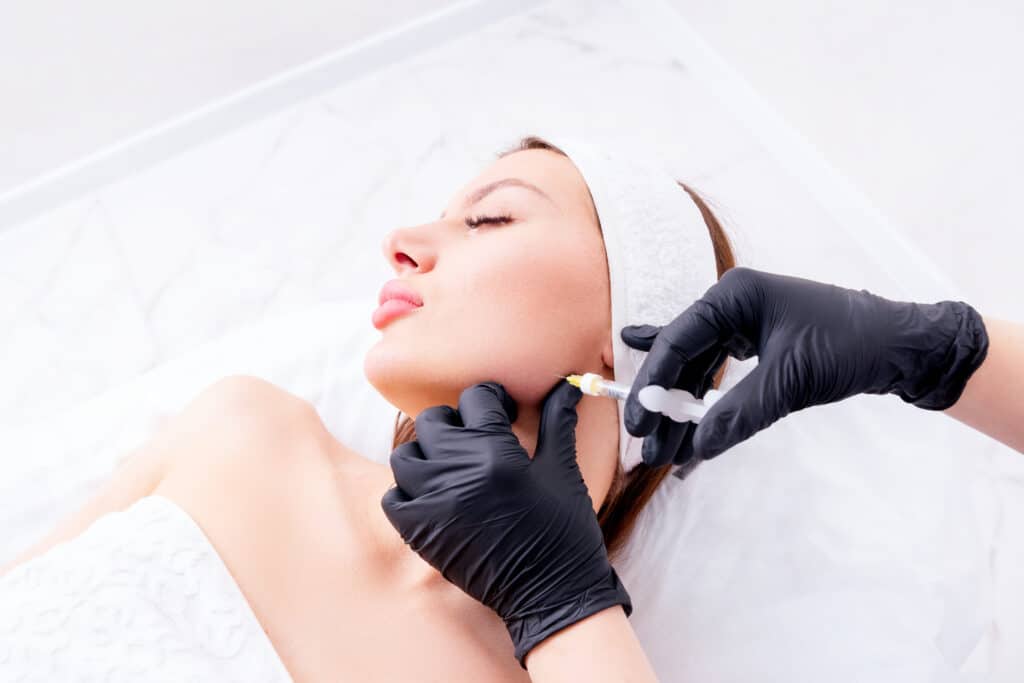
There is a new dermal filler on the market! Juvéderm’s® newest filler, Volux™, has been approved by the FDA and is set to hit medical offices in early 2023. This new filler is designed to specifically target the jawline, an area of concern for many consumers seeking facial sculpting and contouring.
With Volux, medical professionals have a new option for providing results and increased support and structure for the cheeks, chin, and jawline. In this post, we’ll explore the specifics of Volux, how it works, and what medical professionals can expect when incorporating it into their aesthetic practice.
Volux for Medical Professionals: What You Need to Know
- Volux is made from a type of hyaluronic acid that provides more durability and support than other fillers, making it ideal for use in areas that require more structure and definition, such as the jawline.
- Volux is the first hyaluronic acid filler to be FDA approved specifically for the jawline, making Volux an option for patients seeking lower facial sculpting and contouring.
How Volux Works: A Technical Look
- Volux is injected deep onto the bone along the jawline and chin to provide more definition and support, with results lasting from a year to a year and a half.
- Patients may experience tenderness, swelling, lumps, and bruising, but these side effects are likely to subside within two weeks.
- Proper aftercare, such as avoiding ibuprofen, fish oils, and aspirin, can help minimize bruising.
Expert Opinions on Volux
- According to Dr. Hadley King, a New York City-based board-certified dermatologist and clinical instructor of dermatology at the Weill Medical College of Cornell University, “Volux has more structure and cohesivity and lift capacity” than other Juvéderm fillers, such as Voluma XC. This increased firmness provides the chiseled jawline that many patients seek.
- Medical professionals should ensure that the face is cleansed with a topical antiseptic prior to the procedure to reduce the risk of infection.
Incorporating Volux into Your Aesthetic Practice
- Training is now available for medical professionals interested in expanding their aesthetic practice, with proper technique and training which is the key to achieving optimal results.
- Patients with a history of multiple severe allergies or prone to allergic reactions to lidocaine or the gram-positive bacterial proteins that are found in this filler should be advised to avoid this treatment.
As a medical professional, it’s important to stay up-to-date on the latest innovations in non-surgical procedures to ensure that you can provide your patients with the best possible results. At Aesthetic Advancements Institute, we offer comprehensive and personalized education programs that provide medical professionals with the knowledge and skills they need to succeed in this rapidly growing field. We’re excited to offer training on all FDA approved neurotoxins and dermal fillers to our students and help them stay at the forefront of the aesthetics industry.
References: